Research
Physical Properties of Ionic Liquids
Physical properties of 4 room-temperature ionic liquids consisting of the 1-butyl-3-methyl-imidazolium cation with various perfluorinated anions and the bis(trifluoromethylsulfonyl)imide (Tf2N-) anion with 12 pyrrolidinium, ammonium, and hydroxyl-containing cations are measured. Electronic structure methods are used to calculate properties related to the size, shape, and dipole moment of individual ions. Experimental measurements of phase transition temperatures, densities, refractive indices, surface tensions, solvatochromic polarities based on absorption of Nile Red, 19F chemical shifts of the Tf2N- anion, temperature dependent viscosities, conductivities, and cation diffusion coefficients are reported. Correlations among the measured quantities as well as the use of surface tension and molar volume for estimating Hildebrand solubility parameters of ionic liquids are also discussed.
Publication related to this research:[PDF]
Solvation and Rotational Dynamics of C153 in Ionic Liquids
Steady-state and time-resolved emission spectroscopy with 25 ps resolution are used to measure equilibrium and dynamic aspects of the solvation of coumarin 153 (C153) in a diverse collection of 21 room temperature ionic liquids. The ionic liquids studied here include several phosphonium and imidazolium liquids previously reported as well as 12 new ionic liquids that incorporate two homologous series of ammonium and pyrrolidinium cations. Steady-state absorption and emission spectra are used to extract solvation free energies and reorganization energies associated with the S0 ↔ S1 transition of C153. These quantities, especially the solvation free energy, vary relatively little in ionic liquids compared to conventional solvents. Some correlation is found between these quantities and the mean separation between ions (or molar volume). Time-resolved anisotropies are used to observe solute rotation. Rotation times measured in ionic liquids correlate with solvent viscosity in much the same way that they do in conventional polar solvents. No special frictional coupling between the C153 and ionic liquid solvents is indicated by these times. But, in contrast to what is observed in most low-viscosity conventional solvents, rotational correlation functions in ionic liquids are non-exponential. Time-resolved Stokes shift measurements are used to characterize solvation dynamics. The solvation response functions in ionic liquids are also non-exponential and can be reasonably represented by stretched exponential functions of time. The solvation times observed are correlated to the solvent viscosity, and the much slower solvation in ionic liquids compared to dipolar solvents can be attributed to their much larger viscosities. Solvation times of the majority of ionic liquids studied appear to follow a single correlation with solvent viscosity. Only liquids incorporating the largest phosphonium cation appear to follow a distinctly different correlation.
Publication related to this research:[PDF]
Heterogeneous Dynamics in Ionic Liquids
The excitation wavelength dependence of emission kinetics of several solutes is measured by steady-state and time-resolved emission spectroscopy and used to demonstrate the presence of dynamic heterogeneity in several room temperature ionic liquids (ILs). The solute kinetics examined here include rotational and solvation dynamics of Coumarin 153, isomerization of two malononitriles, trans-2-[4-(dimethylamino)styryl]benzothiazole isomerization, and intramolecular electron transfer in crystal violet lactone. The rates of most of these processes vary significantly with excitation wavelength, especially from excitation on the red edges of the solute absorption bands, indicating that energetically selected subpopulations relax at distinct rates. The results presented here suggest more generally that dynamic process taking place on the sub-nanosecond time scale in typical ionic liquids near room temperature are likely to be heterogeneous in character. Similar heterogeneous dynamics are also observed in low temperature 1-propanol, which indicates dynamic heterogeneity is not a unique character of ILs.
Publication related to this research: [PDF]
Non-Radiative Deactivation of Malononitriles
Push-pull molecules 4-N,N-dimethylamino-benzylidenemalononitrile(DMN) and joulolidine-malononitrile (JDMN) have long been examined for their non-linear optical properties and as microviscosity probes in conventional solvents, polymers, biological systems and recently ionic liquids. In ths study, malononitriles are studied in a collection of conventional room-temperature solvents with different viscosities and polarities by both steady-state and time-resolved emission spectroscopy. Both the absorption and emission spectra of DMN and JDMN show large shifts with solvent polarity and these shifts are reasonably correlated to dielectric properties. The quantum yields of malononitriles are sensitive to solvent viscosity but the relationship is not simple. The quantum yields of malononitriles do not seem to be sensitive to solvent polarity. Our quantum yields do not agree with previous reported values, but the decay times deduced from them are consistent with time-resolved measurements from TCSPC and Kerr-gated technique. Temperature dependent data on malononitriles in 2-methyltetrahydrofuran and 1-propanol over a wide temperature range are also presented. The temperature dependence of the emission of malononitriles in MTHF and PrOH provides further information about non-radiative deactivations. We observe an unusual behavior of spectral shifts with temperature, which result from different temperature dependence of the solvation time and lifetime. Reaction times correlate with 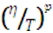 with p values of 1.0, 0.60 and 0.44 for MTHF, PrOH and [Nip311+][Tf2N-] respectively. The smaller values of p, thus the weaker correlation between reactive motion and viscosity, appear to be related to the complexity of the solvation dynamics over the temperature range observed. The detailed spectral dynamics of DMN at low temperature are complex due to the interplay between solvation and reaction dynamics.
with p values of 1.0, 0.60 and 0.44 for MTHF, PrOH and [Nip311+][Tf2N-] respectively. The smaller values of p, thus the weaker correlation between reactive motion and viscosity, appear to be related to the complexity of the solvation dynamics over the temperature range observed. The detailed spectral dynamics of DMN at low temperature are complex due to the interplay between solvation and reaction dynamics.
Publication related to this research: Hui Jin, Chet Swalina, Sergei Arzhantsev, and Mark Maroncelli, Characterization of Malononitriles as Local Fluidity Probes, in preparation for J. Phys. Chem. B